 Nanofiltration is a relatively recent membrane process used most often with low total dissolved solids water such as surface water and fresh groundwater, with the purpose of softening (polyvalent cation removal) and removal of disinfection by-product precursors such as natural organic matter and synthetic organic matter.
Nanofiltration is a relatively recent membrane process used most often with low total dissolved solids water such as surface water and fresh groundwater, with the purpose of softening (polyvalent cation removal) and removal of disinfection by-product precursors such as natural organic matter and synthetic organic matter.
Nanofiltration (NF) is one of the four membrane technologies, which utilize pressure to effect separation of contaminants from water streams. The other three are microfiltration, ultrafiltration and reverse osmosis (RO). All of these technologies utilize semi-permeable membranes that have the ability to hold back (reject) dissolved and/or suspended solids from a water stream containing these contaminants.
Nanofiltration is also becoming more widely used in food processing applications such as dairy for simultaneous concentration and partial (monovalent ion) demineralization.
This mechanism depends upon the valence of the salt ion in question. Recognize that a salt is a compound of two or more ions with an electronic charge. Valence is the number of charges on the ions that form the specific salt, which is not always sodium chloride (NaCl); sodium and chloride are monovalent ions because they have only one charge, whereas ions such as calcium and sulfate are multivalent because they have more than one charge. A defining characteristic of NF membranes is that they reject multivalent ions to a significantly greater degree than monovalent ions. The specific rejection of ions varies from one membrane manufacturer to another, but a multivalent ion rejection of 95 percent with a monovalent ion rejection of only 20 percent isn’t unusual for NF membranes.
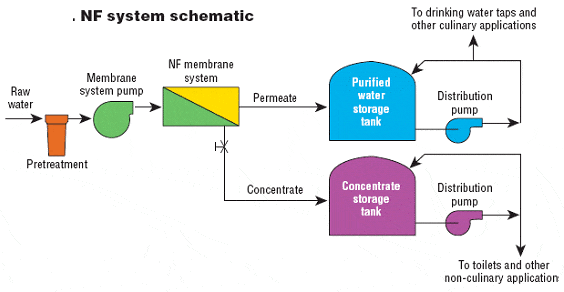
Most of these membranes available today are in spiral wound construction only, although it’s expected that capillary fiber nanofilters will soon be on the market. Figure 1 illustrates NF in terms of its removal efficacy.
In much of the developing world, clean drinking water is hard to come by, and nanotechnology provides one solution. While nanofiltration is used for the removal of other substances from a water source, it is also commonly used for the desalination of water. As seen in a recent study in South Africa, tests were run using polymeric nanofiltration in conjunction with reverse osmosis to treat brackish groundwater. These tests produced potable water, but as the researchers expected, the reverse osmosis removed a large majority of solutes. This left the water void of any essential nutrients (calcium, magnesium ions, etc.), placing the nutrient levels below that of the required World Health Organization standards. This process was probably a little too much for the production of potable water as researchers had to go back and add nutrients to bring solute levels to the standards levels for drinking water. On another note, providing nanofiltration methods to developing countries, to increase their supply of clean water, is a very inexpensive method compared to conventional ones. However, there remain issues as to how these developing countries will be able to incorporate this new technology into their economy without creating a dependency on foreign assistance.
To dissolve air for flotation, three types of pressurized systems are used. Full-flow or total pressurization is used when the wastewater contains large amounts of oily material. The intense mixing occurring in the pressurization system does not affect the treatment results. Partial-flow pressurization is used where moderate to low concentrations of oily material is present. Again, intense mixing by passage through the pressurization systems does not affect treatment efficiency significantly. The recycle-flow pressurization system is for treatment of solids or oily materials that would degrade by the intense mixing in the other pressurization systems. This approach is used following chemical treatment of oil emulsions, or for clarification and thickening of flocculent suspensions.
In the schematic drawing of dissolved-air flotation system shown in the figure, The solids-laden or oily-water influent mixture enters the flotation vessel, and the air-solids mixture rises to the liquid surface. The air-solids mixture has a specific gravity less than water. Solids having a specific gravity greater than water tend to settle to the bottom and are removed by a rotating scraper arm. Attached to the same shaft is a rotating skimmer blade that removes the floating matter from the surface of the vessel into a skimming hopper. Clean water passes underneath a skirt and then must leave the vessel through a launder, which is located in the peripheral region.
Some typical applications for Nanofiltration are:
- Desalination of food, dairy and beverage products or byproducts
- Partial Desalination of whey, UF permeate or retentate as required
- Desalination of dyes and optical brighteners
- Purification of spent clean-in-place (CIP) chemicals
- Color reduction or manipulation of food products
- Concentration of food, dairy and beverage products or byproducts
- Fermentation byproduct concentration.
Nanofiltration and softening : Water softening generally involves the removal of hardness ions, specifically calcium and Magnesium. Because these ions are multivalent, they’re preferentially removed by NF membranes.
As a matter of fact, NF has been used for a number of years for municipal softening, particularly in Florida. The advantage of NF over RO, the other membrane technology that rejects ions, is that NF has a higher flux rate. This means that fewer membrane elements are required and it operates at a lower pump pressure—pounds per square inch (psi) or bars—thereby offering savings in operating costs.
The particular advantage of membrane technology in this application is that no chemicals are required to facilitate the removal of hardness ions, whether soda lime for municipal softening or common salt (sodium chloride) in the case of regeneration of typical residential water softeners. Sodium ion exchange, the standard technology for residential water softening for more than 50 years, utilizes ion exchange resin (in the sodium form) that adsorbs hardness ions from water passing through a bed of such resin, and releases sodium ions in exchange. Because this technology requires sodium or potassium chloride for regeneration of the resin, these are released into the sewer (or septic tank) with every regeneration cycle.
Recent legislation has been enacted to limit these discharges, based upon concerns ranging from excessive chlorides to total dissolved solids (TDS) contamination,
and it appears that an increasing number of communities will prohibit the installation of traditional residential water softeners in the future.
